Abstract
BackgroundTP53 mutations (TP53mt) are associated with dismal outcomes in myelodysplastic syndromes (MDS). However, clinical and biological heterogeneity among TP53mt MDS patients (pts) exists, including the prognostic relevance of mono- vs biallelic TP53mt. The newly proposed WHO 2022 and ICC 2022 classifications recognize TP53mt as a unique entity but vary in its definition. We aimed to assess the impact of TP53 allelic status and complex cytogenetics in different clinical subsets of TP53mt MDS.
Methods A total of 2355 MDS pts with annotated clinical and molecular data treated at Moffitt Cancer Center were analyzed. We defined MDS with biallelic TP53 inactivation (biTP53) according to the 5th edition of the WHO classification. Biallelic status was determined by VAF ≥50%, 2 or more TP53mt and/or TP53mt with deletion 17/17p abnormalities. Pts were classified into IPSS-R lower risk (LR) and higher risk (HR) strata by merging IPSS-R VL, L and I categories, as well as H and VH groups.
Results A total of 490 pts (21%) harbored a TP53mt. Of these, 78% (382) were biTP53 and 22% (108) maTP53. Median duration of follow-up was 4.7 years (4.4-4.9). Pts harboring TP53mt had significantly shorter OS relative to their TP53wt counterparts: median OS 1.2 yrs vs 4 yrs (p<.001). Median OS was worse for the biTP53 subset compared to maTP53: 1.0 yrs (0.9-1.1) vs 1.3 yrs (1.1-1.6; p<.001). Compared to those harboring TP53wt, pts with maTP53 had double the risk of death: HR 2.1 (1.8-2.5). For pts with biTP53 the risk quadrupled: HR 4.0 (3.4-4.7). Both were significant at p<.001.
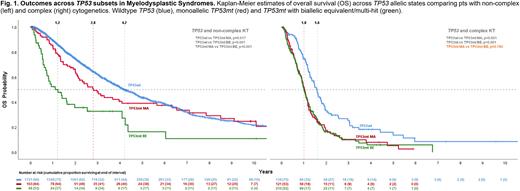
Patients harboring complex (CK) vs non-complex (nCK) karyotypes had inferior mOS of 1.0 yrs (0.9-1.1) vs 2.1 yrs (1.5-2.7; p<.001). Compared to TP53wt, the risk of death was higher for TP53 with CK [HR 4.7 (4.1-5.4)] than for TP53 with nCK [HR 1.5 (1.2-1.8)], both significant at p<.001. Among patients with nCK, allelic state had significant impact on outcomes: mOS for maTP53 was 2.8 yrs (2.0-3.6) vs 1.2 yrs (0.6-1.8) for biTP53 (p<.001; Figure 1). Compared to TP53wt, HR for death for maTP53 was 1.2 (0.9-1.5, p=.21) and for biTP53 was 2.7 (1.9-3.8, p<.001). There was no survival distinction between maTP53 and biTP53 among pts with CK (p=0.780; Figure 1). Both had mOS of 1 yr (0.9-1.2) and were associated with worse outcomes compared to CK TP53wt (p<0.001).
Among pts with LR disease, outcomes were significantly worse for those harboring TP53mt: mOS for TP53wt was 5.2 yrs (4.7-5.7) compared to 3.3 yrs (1.6-5.0, p<.001) for maTP53 and 2.1 yrs (0-4.4, p<.001) for biTP53. There were no differences between maTP53 and biTP53 (p=.2). Allelic status remained significant in HR disease (p=.033), where mOS was worse for biTP53 compared to maTP53: 0.9 yrs (0.8-1.0) vs 1.1 yrs (1.0-1.2).
Similarly, allelic state had value in pts with <5% and ≥5% blasts. Among pts with <5% blasts, TP53wt mOS was 4.8 yrs (4.3-5.4) vs 2.5 yrs (1.7-3.3) for maTP53 and 1.3 yrs (0.9-1.7) for biTP53 (p=.032). Outcomes were worse among TP53mt pts with ≥5% blasts: mOS for TP53wt was 1.7 yrs (1.6-1.9), with distinction between maTP53 and biTP53: 1.2 yrs (1.0-1.2) and 0.9 yrs (0.8-1.0) respectively (p=.005). For the <5% group, the median VAF in maTP53 was 19.5% and 40% in biTP53. For the ≥5% group, the median VAF in maTP53 was 24% (95) and 47.3% (158) in biTP53. There were no differences in outcomes using 10% blast burden as cutoff.
ConclusionTP53mt MDS demarcates a heterogenous group, whose behavior is influenced by allelic status and cytogenetic architecture. TP53mt patients have poor prognosis with ≥5% blasts without distinction from a 10% cutoff. In TP53mt disease, allelic state has strong significance in pts with non-CK, and retains significance independent of IPSS-R strata and myeloblast percentage, albeit with marginal differences. Notably, CK matters independent of allelic status and is invariably associated with worse outcomes. TP53mt with CK should homogenously be considered a biallelic equivalent. This has important implications for future trial design where distinction of allelic state and CK carries relevance.
Disclosures
Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Sweet:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AROG: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Syntrix Pharmaceuticals: Research Funding; berGenBio: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Curis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Mablytics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Lancet:Agios/Servio: Consultancy; Syntrix Pharmaceuticals: Research Funding; Jazz: Consultancy; Astellas: Consultancy; Boxer Capital: Consultancy; Jasper Therapeutics: Consultancy; Dedham Group: Consultancy; Novartis: Consultancy; Dava Oncology: Consultancy; BerGenBio: Consultancy; Millenium Pharma/Takeda: Consultancy; ElevateBio Management: Consultancy; Daiichi Sankyo: Consultancy; Celgene/BMS: Research Funding; AbbVie: Consultancy; Servier: Consultancy. Padron:Syntrix Pharmaceuticals: Research Funding; BMS: Research Funding; Taiho: Honoraria; Incyte: Research Funding; Blueprint: Honoraria; Stemline: Honoraria; Kura: Research Funding. Komrokji:Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Honoraria, Other, Speakers Bureau; CTI biopharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servio: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Taiho: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sallman:Takeda: Consultancy; AbbVie: Membership on an entity's Board of Directors or advisory committees; Aprea: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau; Shattuck Labs: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Intellia: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Magenta: Consultancy; Nemucore: Membership on an entity's Board of Directors or advisory committees; Syntrix Pharmaceuticals: Research Funding; Lixte: Patents & Royalties: LB-100.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal